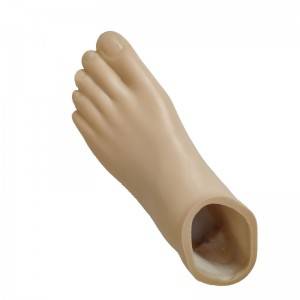
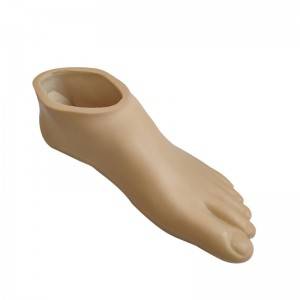
Beige Foot cover
| Product name | Beige Foot cover |
| Item NO. | 1F10SE |
| Color | Beige |
| Size Range | 22~28cm |
| Product weight | 280-500g |
| Load range | 100kg |
| Material | Polyurethane |
| Application | prosthetic foot parts,Foot cover is used with carbon fiber foot. |
Company Profile
.Business Type : Manufacturer
.Main products:Prosthetic parts, orthotic parts
.Experience:More than 15 years.
.Management system:ISO 13485
.Location:Shijiazhuang, Hebei, China.
- Processing Steps:
Drawings design—Mold making—Precision casting—CNC maching—Polishing—SurfaceFinishing—Assembly—Quality Inspection—Packing—Stock—Delivery
- Certificate:
ISO 13485/ CE/ SGS MEDICAL I/II Manufacture certificate
- Applications:
For prosthesis; For orthotic; For paraplegia; For AFO brace; For KAFO Brace
- Main Export Markets:
Asia; Eastern Europe; Mid East; Africa; Western Europe; South America
㈠ Cleaning
⒈ Clean the product with a damp,soft cloth.
⒉ Dry the product with a soft cloth.
⒊ Allow to air dry in order to remove residual moisture.
㈡ Maintenance
⒈A visual inspection and functional test of the prosthetic components should be performed after the first 30 days of use.
⒉Inspect the entire prosthesis for wear during normal consultations.
⒊Conduct annual safety inspections.
CAUTION
Failure to follow the maintenance instructions
Risk of injuries due to changes in or loss of functionality and damage to the product
⒈ Observe the following maintenance instructions.
㈢ Liability
The manufacturer will only assume liability if the product is used in accordance with the descriptions and instructions provided in this document.The manufacturer will not assume liability for damage caused by disregarding the information in this document,particularly due to improper use or unauthorised modification of the product.
㈣ CE conformity
This product meets the requirements of the European Directive 93/42/EEC for medical devices.This product has been classified as a class I device according to the classification criteria outlined in Annex IX of the directive.The declaration of conformity was therefore created by the manufacturer with sole responsibility acording to Annex VLL of the directive.
㈤ Warranty
The manufacturer warrants this device from the date of purchase.The warranty covers defects that can be proven to be a direct result of flaws in the material,production or construction and that are reported to the manufacturer within the warranty period.
Further information on the warranty terms and conditions can be obtained from the competent manufacturer distribution company.