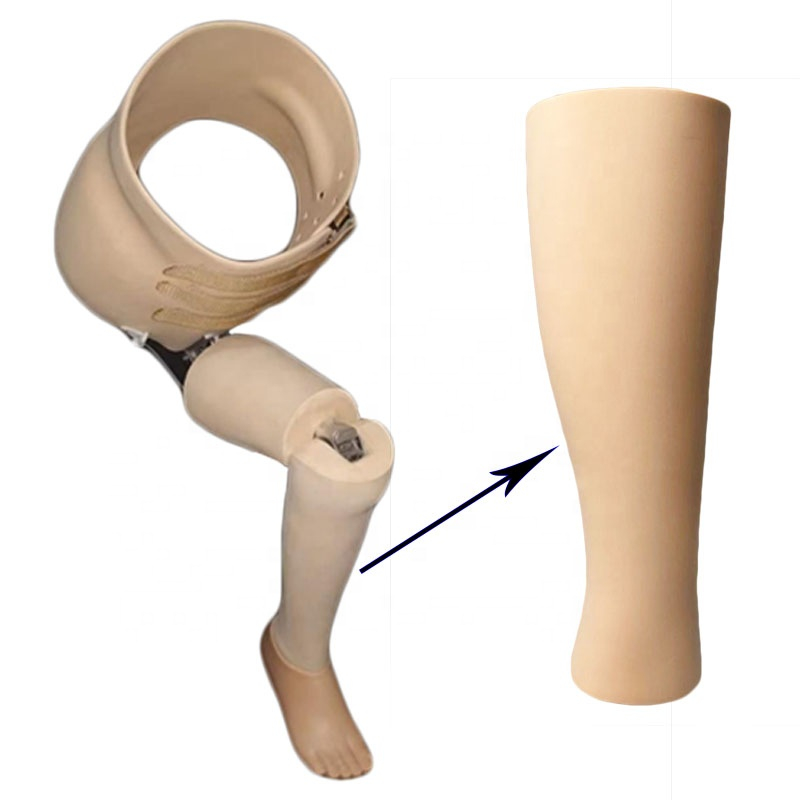
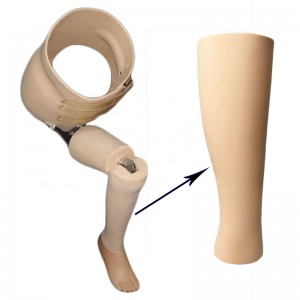
BK Cosmetic Foam Cover(Water Proof)
| Product name | BK Cosmetic Foam Cover(Water Proof) |
|
Item NO.
|
BKFC-6F19 |
| Color | BEIGE |
| Size Range | M/L |
| Product weight | 150 g |
| Load range | 100-125 kg |
| Material | PE FOAM |
| cosmetic foam cover | BK |
| Main Kinds | AK foam cover :AK Cosmetic Foam Cover(Ordinary) ,AK Cosmetic Foam Cover(Strong),AK Cosmetic Foam Cover(Water proof) BK foam cover :BK Cosmetic Foam Cover(Ordinary) ,BK Cosmetic Foam Cover(Strong) ,BK Cosmetic Foam Cover(Water proof) ,AK Cosmetic Foam Cover(Pre-shape) |
Suitable for thigh or calf amputees:
The elasticity of the inner sleeve makes it soft and tightly contain the stump, protect and stabilize the soft tissue of the stump, avoid the friction of the stump in the receiving cavity, reduce or even eliminate local pressure, and provide a comfortable cushion for the sensitive area of the skin. When walking, it can significantly relieve the friction and pressure on the skin surface, reduce pain and wear, and make the amputee feel very comfortable.
Daily wear of lower limb prostheses means that the residual limb will be placed in the receiving cavity for a long time. Due to the sealing of the cavity and the normal metabolism of the body, the receiving cavity will be humid, high temperature, pollution and adhesion. If the skin has adverse effects, it is necessary to develop the habit of cleaning and checking the receiving cavity regularly.
The absorption cavity that directly contacts the skin needs to be cleaned and disinfected every day. You can wipe it from the inside out with 75% alcohol or soapy water, and then dry it. The receiving cavity with inner liner or liner should be cleaned and disinfected regularly. The liner or liner can be removed, sprayed with 75% alcohol or washed with soapy water, cleaned and dried.
Company Profile
.Business Type: Manufacturer/Factory
.Main products: Prosthetic parts, orthotic parts
.Experience: More than 15 years.
.Management System:ISO 13485 .Certificate: ISO 13485/ CE/ SGS MEDICAL I/II Manufacture certificate
.Location: Shijiazhuang, Hebei, China.
.Advantage: Comlete kinds products,good quality,excellent price,best after-sales service,and specially we have ourselves design and development teams,all the designers have rich experienced in prosthetic and orthotic lines.So we can provide professional customization(OEM service) and design services(ODM service) to meet your unique needs.
.Business Scope: Artificial limbs, orthopedic devices and related accessories needed by medical rehabilitation institutions. We mainly deal in the sale of lower limb prosthetics, orthopedic appliances and accessories,materials, such as artificial feet, knee joints, locking tube adapters, Dennis Brown splint and cotton stockinet, glass fiber stockinet, etc. And we also sell prosthetic cosmetic products, such as foaming cosmetic cover(AK/BK), decorative socks and so on.
.Main Export Markets: Asia; Eastern Europe; Mid East; Africa; Western Europe; South America
Packing
.The products firstly in a shockproof bag, then put into a small carton, then put into a normal dimension carton, Packing is suitable for the sea and air ship.
.Export carton weight: 20-25kgs.
.Export carton Dimension: 45*35*39cm/90*45*35cm
Payment and Delivery
.Payment Method :T/T, Western Union, L/C
.Delivery Tiem: within 3-5 days after receiving the payment.
Maintenance
1.A visual inspection and functional test of the prosthetic components should be performed after the first 30 days of use.
2.Inspect the entire prosthesis for wear during normal consultations.
3.Conduct annual safety inspections.
Caution
Failure to follow the maintenance instructions
Risk of injuries due to changes in or loss of functionality and damage to the product
Liability
The manufacturer will only assume liability if the product is used in accordance with the descriptions and instructions provided in this document.The manufacturer will not assume liability for damage caused by disregarding the information in this document,particularly due to improper use or unauthorised modification of the product.
CE conformity
This product meets the requirements of the European Directive 93/42/EEC for medical devices.This product has been classified as a class I device according to the classification criteria outlined in Annex IX of the directive.The declaration of conformity was therefore created by the manufacturer with sole responsibility acording to Annex VLL of the directive.
Warranty
The manufacturer warrants this device from the date of purchase.The warranty covers defects that can be proven to be a direct result of flaws in the material,production or construction and that are reported to the manufacturer within the warranty period.
Further information on the warranty terms and conditions can be obtained from the competent manufacturer distribution company.